Allopathic medicine for acidity became the cause of cancer
On

'Bhai Rakesh 'Bharat', Co-Editor and
Chief Central Incharge Bharat Swabhiman
-
Carcinogen drugs that were removed from the market
-
Stomach gas, acidity removal medicine can also make you a cancer patient
India when a patient goes to any doctor in India or all over the world for any disease, in 90% of the cases the doctor first prescribes a medicine before prescribing the medicine for that disease, about which it is mentioned goes with the medicine you are taking you have to take this medicine ofacidity medicine only.
The medicine which you consider to be very safe and buy it from a chemist of a good brand after getting it prescribed on the advice of a very well-educated city doctor, that medicine can also give a disease like cancer, it may sometimes be caused by an allopathic medicine. The patient doing the experiment would not have imagined even in his dreams?
Whenever we eat any modern allopathy medicine, which is not familiar with the internal system of our body, our body tries to take it out considering it as a foreign material and tries to take it out. In this, our digestive system has to load on our liver to digest it, to metabolize it, and in this process, the first reaction the body gives is that of acidity, to suppress the alarm system of this acidity, the doctor gives us any Medicines with antacid effect are given to control acidity while giving types of antibiotics, pain killers or other treatments. All these medicines by pharma companies making big claims, big-
By publishing research in a big research paper and declaring it 100% safe, 1 to 4 doses are fed several times a day for a few weeks and sometimes continuously.
How millions of people control acidity drug which caused cancer
An English chemist named John Bradshaw first prepared it as AH19065 in 1976 at the Ware Research Laboratories of Allen & Humbers Pharma Company. Allen & Humbers Pharma Company and its lab is now part of GlaxoSmithKline. The drug was first launched in the United Kingdom in November 1976 as Tagamet, to treat ulcers in the stomach and intestines and prevent recurrence, caused by acid reflux or reflux disease. A lot of publicity has been given for the treatment of stomach and esophageal problems. Names of H2 blockers to ranitidine also know as. It reduces the amount of acid in the stomach. Due to which ulcer, acidity is felt less. Doctors advised and allowed to use it up to four times in 1 day, it was widely publicized to eat this medicine from morning to evening after dinner or even before sleeping. .
Doctors began prescribing ranitidine for increasing children weight, and to everyone without hesitation. Its name has been included in the list of essential medicines of the World Health Organisation. With 20 million prescriptions in the US alone in 1918, the drug became the 41st most prescribed drug in the US. In America, it is widely prescribed by doctors at the behest of pharma companies for the treatment of gas, acidity, constipation and gastric ulcer under the name of Zantec.
Cancer from ranitidine which was considered safe
In September 2019, Joseph L. Galimidi, a resident of the US state of Florida, sued Sanofi Pharma Company for breast cancer. Joseph L. Galimidi had some health problems, he went to his family doctor and he prescribed some medicines and told that it is necessary to take this medicine to control acidity along with these medicines. Joseph L. Galimidi asked the doctor if this medicine is safe. Citing a well-known company in the research report, the doctor said that this medicine is completely safe. Feel free to consume it. Ranitidine was consumed by Joseph L. Galimidi from 2009 to 2013. Suddenly in 2013 she got breast cancer. Breast cancer, however, is rare in men. When Joseph L. Galimidi was diagnosed with breast cancer, a rare disease for men, he could not understand the reason. He used allopathic medicines as the cause of his cancer. He tried to find out at different places what was the cause of his cancer. From the pharma company to the doctors assured them that the medicine is absolutely safe.
By 2019, they came to know that a substance named 'NDMA' was found in 'Ranitidine', which causes cancer. He went to court and filed the first lawsuit against Sanofi Pharma Company claiming that ranitidine drug caused cancer. He said in the complaint that the burning sensation in his chest was cured by ranitidine but the NDMA found in ranitidine yes, it is carcinogenic and because of that she got breast cancer. Further, he said in his complaint that such reports of Zantec and other ranitidine drugs being sold in the US were coming for many years, but the pharma company did not do anything about it. Did not study or investigate nor gave any kind of warning to the public. If the pharma company had stopped such medicines by warning and checking in time, then people's lives could have been saved from cancer.
Similarly, on October 21, 2019, Mark Allen Black, a resident of Colorado, filed his Gentech lawsuit against Sanofi US Services, Chatham Inc., Boehringer Ingelheim, Pfizer and GlaxoSmithKline, in this lawsuit Mark Allen said that on the advice of the doctor I started taking Gentec in 1996, even after that he continued to take this drug as a generic drug. He used this medicine for 4 days a week and after two decades when he started having severe pain in his stomach and started bleeding in the urine. Then he got his complete checkup done and after getting the test done, he came to know that he has got bladder cancer. He also used to live a healthy lifestyle but he got cancer from the drug called Gentec which he took in the name of antacid. Mark Allen Black clearly wrote in his complaint that research has shown that acidity medicines such as Gentec and other ranitidine products have been producing very high levels of NDMA since 1981, and it is because of this NDMA that he developed bladder cancer. Has happened. He also said that from 1980 to 2019, pharma companies had full time to do research. They should have warned the patients after examining its harmful side effects, but for their own benefit, the pharma company continued to make people cancer patients by getting doctors to prescribe such medicines without any hesitation.
What is NDMA (N-Nitrosodimethylamine)?
The National Library of Medicine states that N-Nitrosodimethylamine is a volatile, flammable, yellow, oily liquid nitrosamine with a characteristic odor. It decomposes on exposure to light and on heating to release toxic fumes of nitrogen oxides. This is the same NDMA that is used in labs to produce tumors in animals used for trials, that is, animals that want to produce cancerous tumors are fed NDMA.
The European Medical Agency believes that NDMA has been proven to cause cancer based on research done on animals. According to a report by the US Environmental Protection Agency (USEPA), NDMA is a carcinogenic agent that causes liver damage first of all. Makes it Other reported side effects of ranitidine are blurred vision, mood changes, severe fatigue, irregular heartbeat, sore throat that does not go away, fever, chills, abdominal pain, dark urine Yellowing of the eyes, itching, swelling, unusual allergic rash on the face, swelling of the throat, dizziness, difficulty in breathing, carcinogenic, and these are other side effects experienced by people taking ranitidine.
Initially, in 2017-18, the FDA found in research that drugs such as ranitidine, when they are stored at temperatures higher than room temperature after production, increase the level of carcinogen NDMA in them, which can affect the person who consumes that drug. Whoever eats it becomes at risk of diseases like cancer. Pharma companies argued that the level of NDMA is only a few increases due to storage mistake in batch medicines, then FDA got its testing and investigation done in third party lab and the results of third party lab came out that even after normal storage, the level of NDMA in ranitidine increases and which is high. When stored at temperature, the level of NDMA increases further.
The 'International Agency for Research on Cancer' also found that NDMA is probably carcinogenic to humans and classified it as a Group 2A carcinogen. They also agreed that we have enough evidence that NDMA can cause cancer in humans and surprisinglyit is that in the side effects which are written on the level of ranitidine, there was no discussion of any kind of side effects regarding cancer.
Drug companies continued to argue that the carcinogenic element in ranitidine is not a part of the drug, but that it is carcinogenic only when the drug is stored at high temperatures in a warm place for a long time, otherwise it is completely is safe.
This claim of pharma companies also turned out to be completely false. Similarly, in March 2021, a research paper was published by Dr. Z. Bronstein and his colleagues in a well-known research journal named JAMA. They said that ranitidine enters the human stomach and interacts with certain conditions. Can create toxic levels of NDMA in the body when ingested. Researchers also found that ranitidine was capable of producing NDMA far beyond the FDA's allowable limit, meaning that the drug was capable of causing cancer due to toxicity.
Although in 2019 the FDA and the European Medical Agency claimed they didn't directly ban Ranitidine, they said that Ranitidine contains chemical called NDMA which can cause cancer and they decided to ban it completely by 2020 only after thoroughly examining it.
Ranitidine banned by various drug regulators
In September 2019, big stores like Walmart, Walgreens etc. in America removed ranitidine tablets from their stores. On 8 October 2019, the United Kingdom's Medicines and Health Care Products Regulatory Agency issued a drug alert for ranitidine to initiate a recall process from UK hospitals, pharmacies and distribution channels and retailers. On 15 October 2019, the United Kingdom's Department of Health issued a warning for all combinations of ranitidine. In November and December 2019 and January 2020, companies ranging from Aurobindo Pharma to Sanofi, Glenmark Pharma etc. started withdrawing their Ranitidine tablets.
After being fully certified in April 2020, the FDA completely barred pharma companies from distributing ranitidine and selling ranitidine over-the-counter in the United States with or without a prescription.
In December 2020, the European Medicines Agency banned all products of ranitidine for human use.
As of December 2021, the United Kingdom's National Health Service (NHS) announced on its website that no products of ranitidine are currently available in the UK. Sunora Active Pharma Sciences, which supplies Ranitidine's API i.e. its raw material, has clarified to the European Medical Agency that it has reduced the risk of NDMA in Ranitidine raw material and that it is complying with all the rules of the European Directive.
The raw material manufacturing companies of Pharma companies again wanted to launch Ranitidine in Europe, America and UK market, but Glaxo Smith Kline, Sanofi, Ateva who are big Pharma companies said that looking at its danger, we will not launch Ranitidine does not agree to the re-production of it.
Since its launch in 1981, Ranitidine has been sold by pharma companies in more than 120 countries to treat 222 million patients worldwide, the largest seller for the treatment of GERD related diseases. After all these investigations, Ranitidine was approved in the United States of America in April 2020 banned in America, European countries and Australia. The British company Galaxo Smith Line voluntarily recalled its best-selling Gentec product worldwide. Similarly, in 2019, Glaxo also removed Ranitidine drug from the markets of India and other countries from the point of view of safety in view of the danger of NDMA.
The name of a drug named Pantoprazole was suggested as an alternative to Ranitidine, but when this drug was also used, it was found that using Pantoprazole weakens the bones. The entire skeletal system is affected and it is also serious for the kidneys is harmful.
Former soldiers file case in the US Department of the Military
Ranitidine was widely used in military hospitals for soldiers and veterans, the Defense Health Agency advised its soldiers to talk to their doctors about alternatives to ranitidine and to educate them about ranitidine cancer risk notice is issued.
At the same time, he said that people who buy over-the-counter without doctor's if you are using ranitidine after purchasing it, stop taking it immediately and report any reaction or side effects to the FDA immediately.
Other cases of ranitidine and their consequences cancer with
According to the FDA's Adverse Event Reporting System, they looked at the total number of side effects reported over the 37 years from 1983 to 2020, and found that the percentage of cancer cases reported is increasing every year. Of the 73,240 cases, 66% reported cancer as a side effect of ranitidine. Side effects reported in 2020, 70% of cancer cases were found due to which 4926 people died.
As of 2020, the FDA has received 15 cancer reports from the common acidity medication ranitidine, including 10,698 cases of colorectal cancer, 7,475 cases of kidney cancer, 6,212 cases of bladder cancer, 4,479 cases of prostate cancer, 4,004 cases of esophageal cancer, stomach 3,099 cases of colon cancer, 2,823 cases of liver cancer, 2,532 cases of pancreatic cancer, 2413 cases of malignant tumors, 2225 cases of lung tumors, 2088 cases of breast cancer, 1125 cases of gastrointestinal cancer, 1031 cases of uterine cancer, testis There were 879 cases of cancer, 697 cases of thyroid cancer.
Deaths linked to ranitidine by 2020
864 cases of pancreatic cancer, 782 cases of liver cancer, 702 cases of esophageal cancer, 675 cases of malignant tumors, 649 cases of colorectal cancer, 608 cases of colon cancer, 455 cases of lung tumor, 373 cases of kidney cancer , 351 cases of bladder cancer, 146 cases of gastrointestinal cancer, 114 cases of prostate cancer, 73 cases of breast cancer, 57 cases of brain tumor, 53 cases of throat cancer, 52 cases of uterine cancer were associated with death.
Ranitidine invited cases against pharma companies
A large number of patients with cancer caused by ranitidine started suing in which they demanded compensation for criminal prosecution Got cancer. No one else in our family has had cancer and we do not have any genetic markers that are carcinogenic.
Cancer has occurred only because of taking acidity medicine. Ranitidine caused urine bladder cancer was the most common.
There are continuous lawsuits on Ranitidine drugs from America to other countries. Judges in courts in California and Florida have given orders that will assess how ranitidine-induced cancer can spread in the future and how much damage it can cause, suing pharma companies for fines, damages and compensation can be determined. Trial underway in California, trial in Florida to begin in July 2023, trial against ranitidine in other US states such as New Jersey, Oregon, New York, Pennsylvania, Tennessee, Texas, Washington etc.
As of August 15, 2022, there are more than 2,000 cases pending against pharma companies in Florida, with thousands of such lawsuits pending in other countries in the US. You should understand the seriousness of these lawsuits, that billions of rupees have to be imposed on them there is bound to be a fine.
Banned drug in the world is being sold openly in India
In India too, ranitidine was promoted and circulated as a popular medicine for acidity, constipation, gas, indigestion etc. Ranitidine was written on prescription to crores of people. Ranitidine is manufactured and sold in India in the form of more than 180 brands of well-known companies from Cadila Pharma to JB Pharma, Dr. Reddy, Sun Pharma, whose main brand names are Aciloc Tablets, Rantec Tablets, Gentec, Pantec. etc. are included. ,
In September 2019, the potential carcinogen N-nitrosodimethylamine (NDMA) was discovered in several manufacturers' ranitidine products. In April 2020, ranitidine was withdrawn from the market in the United States and banned in the European Union and Australia for being carcinogenic. Our neighboring country Bangladesh has also banned this medicine.
To say in India, Ranitidine is considered a drug under Schedule H, that means a doctor's prescription is required to buy it, but you can easily buy this medicine online or from the nearest chemist without any prescription at most drug stores in the country can buy from the key shop.
When the FDA came to know about the serious side effects of ranitidine, they issued a warning and issued letters to the drug regulatory drug controller departments of different countries, giving information about the cancer factor called ranitidine produced in ranitidine warned about the danger. In 2019, a warning was issued by the US. The Drug Controller General of India (DCGI) of the Government of India took it for granted.
When the news of cancer caused by ranitidine spread rapidly all over the world, the Drug Controller General of India was also under pressure to take some action. Instead of immediately banning the banned drug all over the world, DGCI wrote a letter to the drug manufacturers and the State Drug Controllers of different states to investigate the carcinogenic element produced in ranitidine and ensure its process so that Patients should be safe.
After being banned all over the world, there was an immediate need to ban this drug in India as well, but instead of banning it, the department fulfilled its responsibility by issuing only a letter.
It is notable that the Drug Controller General of India, Government of India, has full authority to ban, ban or approve any drug, but instead of banning a harmful drug, only a warning was issued and the state governments Or the responsibility was put on the manufacturers. Most of the state governments do not have any such lab in which it can test the cancer-causing impurity NDMA in the medicine, which is a cancer-causing agent produced in ranitidine.
Many senior doctors and aware people also criticized this move of DGCI and said that instead of issuing only a letter when the drug is banned in other countries, DGCI banned it in Bangladesh. A temporary ban should have been imposed immediately after conducting proper investigation in the country. It is worth mentioning that there are more than 180 products based on ranitidine in India with a sale of about 750 crores. France's drug safety regulator has recalled all brand name and generic ranitidine available in pharmacies and asked manufacturers to stop production.
Health Canada asked all companies to stop distributing ranitidine because 'current evidence suggests that ranitidine may contain NDMA, regardless of the company making it. Singapore's Health Sciences Authority (HSA) tested locally distributed brands of ranitidine and found unacceptable levels of NDMA, prompting a ban on eight brands including GlaxoSmithKline.
The Italian Drug Agency also recalled ranitidine-based drug brands, including those from an Indian company also included are products made from the active ingredient.
Furthermore, the Ministry of Health and Prevention of the Arab country UAE and the Ministry of Health and Community Protection of Saudi Arabia have suspended the registration, import and distribution of all medicines containing ranitidine as a precautionary measure. India's Dr. Reddy's has voluntarily recalled all its ranitidine products from the market as a precautionary measure. Global suppliers such as Sandoz, the generic arm of Novartis, and GlaxoSmithKline (GSK) are among the list of manufacturers who have voluntarily withdrawn the drug as a precautionary measure. For those seeking ranitidine again, the FDA in the US recommends that they manage their heartburn by changing their diet and lifestyle for heartburn and general acidity. Lifestyle changes are more beneficial than medication.
This cancer-causing drug has been banned by conscious countries all over the world, but in India, while taking action on it in 2022, it was only removed from India's National List of Essential Medicines.
Even after being removed from the list of essential medicines, this medicine is easily available in India with different chemists of the country. If you go and say that you want ranitidine, they will easily give you a strip of 40 tablets which costs only ₹30. Data available with the Pharma Traders Association of Indian Origin Chemists and Distributors Ltd suggests that despite the worldwide ban, sales of ranitidine declined by only 2% between August 2021 and August 2022.
Many doctors of India still without any hesitation write the ban cancer causing drug all over the world on patient's prescription and it is easily available all over India. without a doctor you can go anywhere and buy this cancer causing medicine for yourself without the advice of a doctor. This allopathy or pharma mafia is so dangerous that for 40 years ranitidine was declared completely safe. In 40 years, after earning billions of dollars, it was found that millions of people had cancer, as an alternative, PPI i.e. Protein Pump Inhibitor drugs were launched and it was said that the drug is safer, beneficial and more effective than the previous drug. More effective. Pantoprazole drug was publicized a lot, but within a few years, it was also found out about Pantoprazole drug that the PPI drug not made by pharma companies, Pantoprazole is also going to cause severe side effects for bones and kidneys.
The saddest episode is that many allopathy doctors and pharma companies and the false scientists who live on their parole keep on saying that ranitidine is a very good medicine for constipation and those who are promoting it in social media or foreign media Hearing the news of cancer due to this, do not trust them. They go so far as to say that as much cancer-causing NDMA is found in these medicines, it is also found in contaminant, beer, meat, fish, shampoo, soap, detergent etc. Fake doctors and scientists wearing God's robes in the name of such devils shamelessly kept telling such medicines non-harmful to the patient for many years.
No pharma company, no research journal or any other scientist or doctor is concerned about our health. For this we have to become aware ourselves. America's FDA has suggested that by not taking any medicine for gas, constipation, indigestion, acidity, heartburn, we should cure ourselves only by changing the diet and changing the routine, this is our Ayurveda, our ancient Indian Knowledge is also called science.
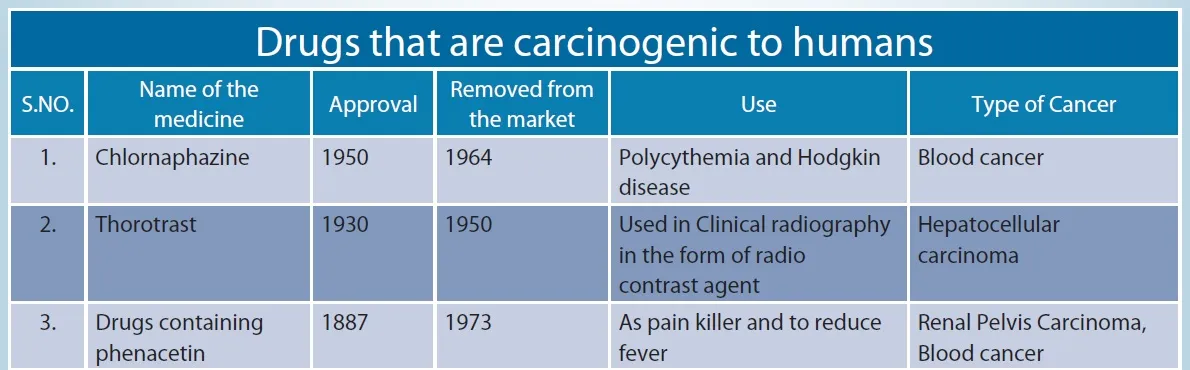
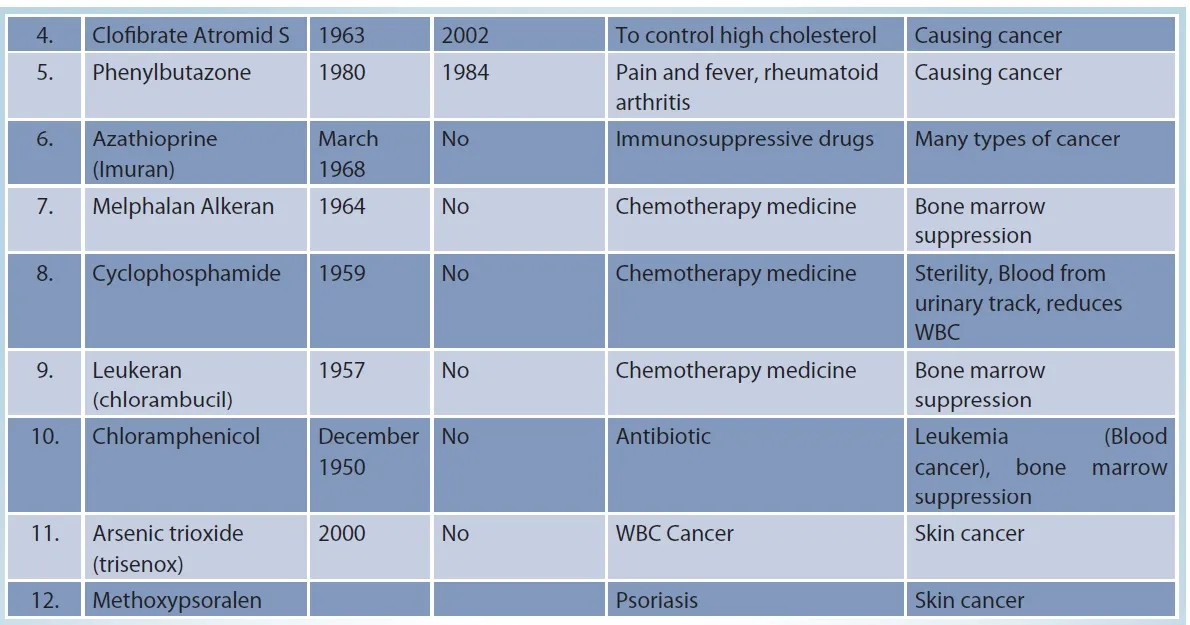
Drugs that are carcinogenic to humans
Source: dol:10.1002/1097-0142(19810301)47:5+<1071::aid-cncr2820471304>3.0.co;2-7, American Cancer Society
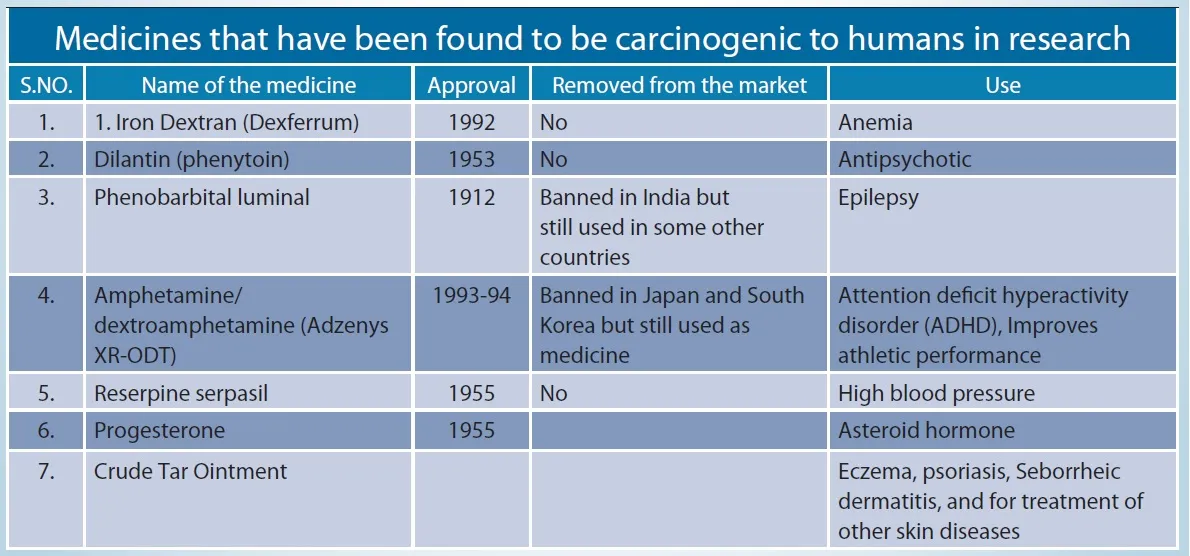
Medicines that have been found to be carcinogenic to humans in research
Source : doi:10.1002/1097-0142(19810301)47:5+<1071::aid-cncr2820471304>3.0.co;2-7, American Cancer Society
Reference:
1. https://www.drugwatch.com/news/2021/03/22/analysis-zantac-form-high-levels-ndma-in-body/
2. https://www.shapirolegalgroup.com/zantac-stat
3. https://www.drugwatch.com/zantac/lawsuits
4. https://pubchem.ncbi.nlm.nih.gov/compound/N-Nitrosodimethylamine
5. https://pharmaceutical-journal.com/article/news/return-of-ranitidine-being-considered-by-uk-manufacturer
लेखक
Related Posts
Latest News
01 Oct 2024 17:59:47
ओ३म 1. सनातन की शक्ति - वेद धर्म, ऋषिधर्म, योग धर्म या यूं कहें कि सनातन धर्म के शाश्वत, वैज्ञानिक,...




.jpg)













.jpg)
.jpg)
